Prism for PTSD is an evidence-based, prescription therapy, FDA-cleared to treat PTSD. During Prism treatment, a computer simulation and EEG headset create an environment where patients engage in a non-trauma-based experience to help them learn to control their PTSD symptoms.
Prism is the first PTSD treatment based on a digital brain-mechanism-specific biomarker associated with mental health disorders.


Clinical studies have demonstrated durable improvement in PTSD symptoms with a high safety profile.
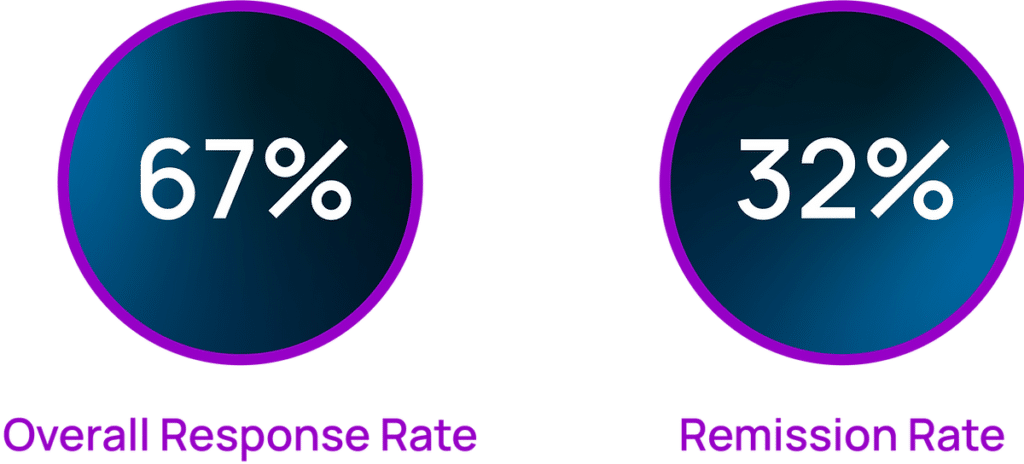
The Prism for PTSD multi-center clinical study demonstrated the following results. 3 months after the 15-session regimen (5 months from baseline):